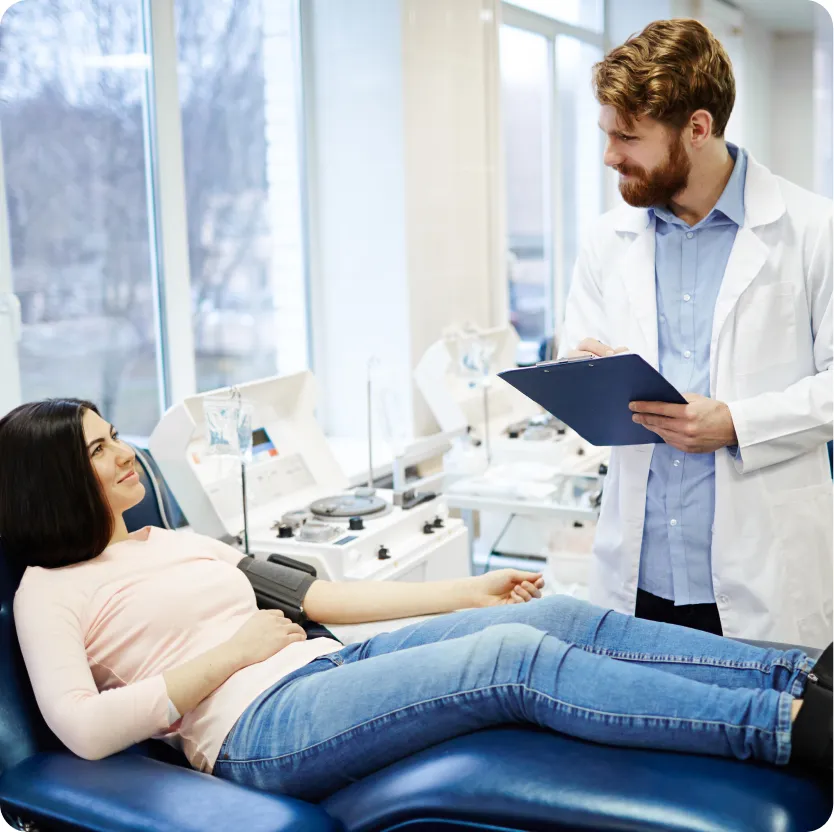
About PPTA
The Plasma Protein Therapeutics Association (PPTA) is a dynamic trade association that represents a unique sector of the biologics and biotechnology industry. PPTA represents more than 1,100 human plasma collection centers in North America and Europe, as well as the manufacturers of lifesaving plasma protein therapies. Our members produce approximately 80% of the plasma protein therapies in the U.S. and 60% of those manufactured in Europe.
What we do
We work globally to advocate for patient access to plasma-derived medicines, responsibly improve the availability of plasma, and advance the understanding of the plasma ecosystem.
Our association administers standards programs that help to ensure the quality and safety of plasma collection and manufacturing, as well as protect both donors and patients.
Mission
Access to therapies
Advocate for Patient Access to Plasma Protein Therapies
- Promote clinician authority and patient participation in treatment decisions
- Pursue reimbursement models recognizing the unique nature of plasma protein therapies
- Shape decisions of authorities to improve plasma protein therapy availability
- Forage partnerships that support patient-focused initiatives

Awareness
Advance the Understanding of the Plasma Ecosystem
- Encourage plasma donation, globally
- Generate and promote evidence-based information about plasma
- Explain the importance, unique nature, and value of plasma protein therapies

Plasma availability
Responsibly Improve the Reliable Availability of Plasma
- Advocate for global sufficiency of plasma
- Pioneer plasma safety and manufacturing quality and support donor well-being
- Drive progressive policies for plasma collections


Mission
European Plasma Alliance
Who we are
This alliance consists of 10 European private sector plasma collectors, operating 137 plasma collection centers:
The EPA’s mission is to promote safe plasma collection practices in the EU with a focus on donor health and donor safety to ensure patients’ access to safe products.
The EPA members currently operate 137 plasma collection centers in four European countries: Germany, Austria, Czech Republic, and Hungary. Last year, EPA members contributed almost 3 million liters of plasma to manufacture lifesaving therapies.
Our goals
Foster plasma collection in Europe
An increasing number of patients across the European Union are diagnosed with life-threatening plasma protein-related disorders, such as Immune Deficiencies, Hereditary Angioedema (HAE), Alpha 1-Antitrypsin Deficiency (AATD), some neurological disorders, or bleeding disorders, such as Hemophilia. Plasma-derived medicinal products (PDMPs) are the primary or, in some cases, the only treatment available for these rare diseases. These developments result in a growing clinical need for PDMPs.
A key element which determines the availability of PDMPs is continuous access to the safe, high-quality starting material, which is human plasma. To meet the growing clinical need of European patients for PDMPs, Europe needs to increase its collection of plasma.To meet this need for increased plasma collection, the EPA advocates for:
Establishing dedicated plasma collection (plasmapheresis) programs and coordinated outreach campaigns toward plasma donors in all European Member States
Allowing the co-existence of public sector and private sector plasma collection centers
Stimulating plasma donations by also allowing compensation of donors for the expenses and inconvenience related to the donation, similar to EU Tissues and Cells Directive (2004/23/EC, article 12.1)
Differentiating between whole blood for transfusion and plasma collection for manufacturing of PDMPs, which is currently lacking in many policy frameworks
These suggestions should be addressed in appropriate policy frameworks at the European and national levels.
Safety is priority
Collecting more plasma from healthy donors in Europe and ensuring adequate patient access to care is stringently linked to preserving donor health.
Plasma donors are saving lives and EPA members are committed to the health and safety of every person who donates plasma
PPTA and EPA support donor health studies
Donors are diverse – Given that the profiles of whole blood donors and source plasma donors differ, “one-size-fits-all” policies are not appropriate
Monitoring of relevant parameters allows tailoring donation intervals & volume.The EPA supports policy decisions regarding donor safety that are based on scientific rationale and data, and which include input from all stakeholders in a fully transparent process.
Contacts
Mag. Milan Maly, European Plasma Alliance, Chairman
Alexa Wetzel, Director, Source Europe
PPTA Europe
This alliance consists of 11 European private sector plasma collectors, operating 137 plasma collection centers:
PPTA France
PPTA France brings together the laboratories Kedrion, Grifols, and Takeda, which specialize in the development and production of blood plasma-derived medicines and recombinant analogs. The association's mission is to contribute to improving the coverage of patients' needs for therapeutic proteins through ongoing dialogue with French health authorities and agencies.


Code of ethics
Our code of ethics ensures the ethical conduct and integrity of the Association. Our members pledge to:

CODE OF ETHICS
Provide the highest quality of therapies, taking into account the health and safety of users of plasma protein therapies (i.e., patients).
Offer therapies to all patients regardless of race, creed, national origin, or source of illness.
Continue to expand and improve the professional knowledge and therapy quality associated with the supply and use of therapies.
Abide by state, national, and multinational laws and regulations that govern the plasma protein therapies and source plasma collection industries, including antitrust and competition laws.
Make best efforts to ensure an adequate supply of safe therapies for medical, pharmaceutical, and scientific use.
Select donors to obtain high quality source plasma while avoiding harmful and/or exploitative treatment of those donors.
Obtain source plasma only from industrialized countries in which a competent national authority is in place to provide assurances of donor and plasma quality, as well as of procedures to avoid harm and/or exploitation of plasma donors.
Support the operation of source plasma programs that include compensation to donors, provided that they operate in such a way as to guarantee the quality of the plasma while avoiding harm and/or exploitation of source donors.
Recognize the ethical responsibility to plasma donors associated with the role of manufacturing therapies of human origin while striving to fulfill the international therapies needs essential in the amelioration of human disease and suffering.
Ensure that advertising and promotions, whether in written, oral, or audio-visual format, shall be truthful, accurate and without misrepresentation.
Agree to act in good faith, and to be honest, truthful and fair to all concerned.
Abide by the laws of the jurisdictions in which member companies operate.
Acknowledge as grounds for denial or termination of a company’s PPTA membership, by action of the Global Board, any illegal action or violation of law that damages the image and credibility of the industry with respect to the safety of therapies, the safety of donors, or truthfulness in scientific exchange.
Acknowledge as grounds for disqualification or removal of an individual from service on a PPTA board, committee, or task force, by action of the Global Board: (1) any illegal action or violation of law that damages the image and credibility of the industry with respect to the safety of therapies, the safety of donors, or truthfulness in scientific exchange; or (2) any act of moral turpitude.
Join PPTA
Become a member of PPTA! PPTA engages with stakeholders to establish and improve regulations and policies that affect the sector.
By being a member of our industry-leading association, you can work closely with influential partners, enjoy numerous benefits and access useful resources.


















Contact us
If you have any inquiries, get in touch with us by filling out our contact form linked below.
Members of the media are encouraged to send press inquiries to media@pptaglobal.org