Plasma Protein Therapeutics Association
about PPTA
The Plasma Protein Therapeutics Association (PPTA) is a key player in the plasma industry, with an extensive network of human plasma collection centers and manufacturers. Collaborating with numerous key stakeholders and policymakers to establish rightful standards and programs for donors and patients is at the core of our work.
Why you should donate plasma
Plasma-derived medicines are often the only therapies for many rare and chronic diseases. By becoming a donor, you increase the chances of regular access to plasma medicines for those in need, contributing to the improvement of health outcomes for patients.

Resource centER
Learn more about our work through the material in our resource center.

Media
Stay up to date with the key developments and issues discussed by our stakeholders, members, and patients by taking a look at our available media resources.
Lemon Williams II Interviewed at PPTA's 2025 Plasma Protein Forum (PPF)
Steve Mitzelfelt Interviewed at PPTA's 2025 Plasma Protein Forum (PPF)
Dr. Sara Del Carlo Interviewed at PPTA's 2025 Plasma Protein Forum (PPF) (English)
Katherine Pajewski Interviewed at PPTA's 2025 Plasma Protein Forum (PPF)
Steven L Spitalnik, M.D. Interviewed at PPTA's 2025 Plasma Protein Forum (PPF)
Dr. Thomas R. Kreil Interviewed at PPTA's 2025 Plasma Protein Forum (PPF)
Dr. Toby Simon's Interview at PPTA's 2025 Plasma Protein Forum (PPF)
Namandjé N. Bumpus, Ph.D., Interviewed at PPTA's 2025 Plasma Protein Forum (PPF)
PPTA's Regulatory Expertise
Working with Regulatory Agencies
Influencing Patient Access Through Regulations
What is Regulatory Policy?
Patient Access to Plasma-Derived Medicines in the U.S.: International Plasma Awareness Week Webinar
The Journey of Plasma-Derived Medicines: From Collection to Care
The Journey of Plasma-Derived Medicines: From Collection to Care (1 min)
Interview with Irene Saugar Gómez, Ph.D., Quality Assessor of biological human medicinal products with the Spanish Agency of Medicines and Medical Devices
PPTA President and CEO Anita Brikman speaks with Grifols Egypt CEO Dr. Magdy Amin
Oksana Muliarchuk - Director of a Ukrainian-Based Network of Plasma Centers
Thomas Kreil, Winner of the Hilfenaus Award
Watch our interview with Prof. Richard Knight as he shares valuable perspectives on immunoglobulins
Patient Stories presented by PPTA
PPTA New Website and Brand Launch
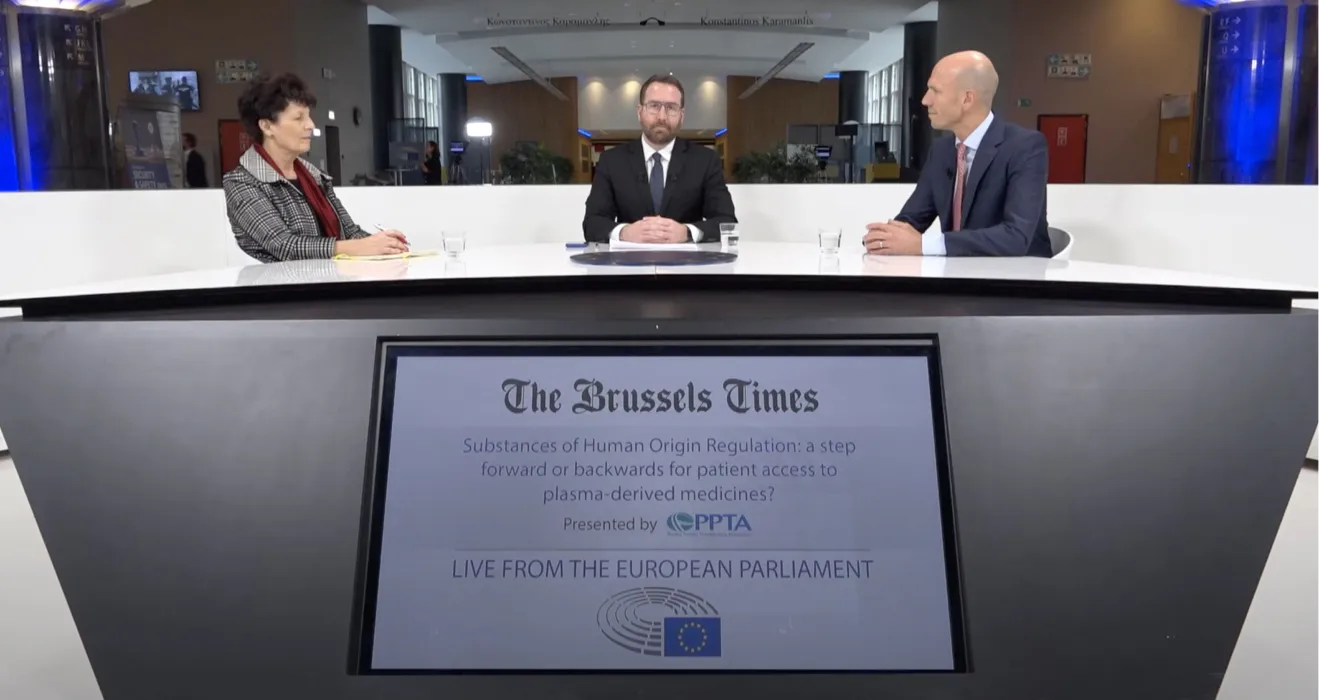
The Brussels Times Hosts Event at the European Parliament
Take a tour of a plasma donation center in Frankfurt, Germany
The need for a policy change – Plasma donation
2022 International Plasma Protein Congress (IPPC): Day One
2022 International Plasma Protein Congress (IPPC): Day Two
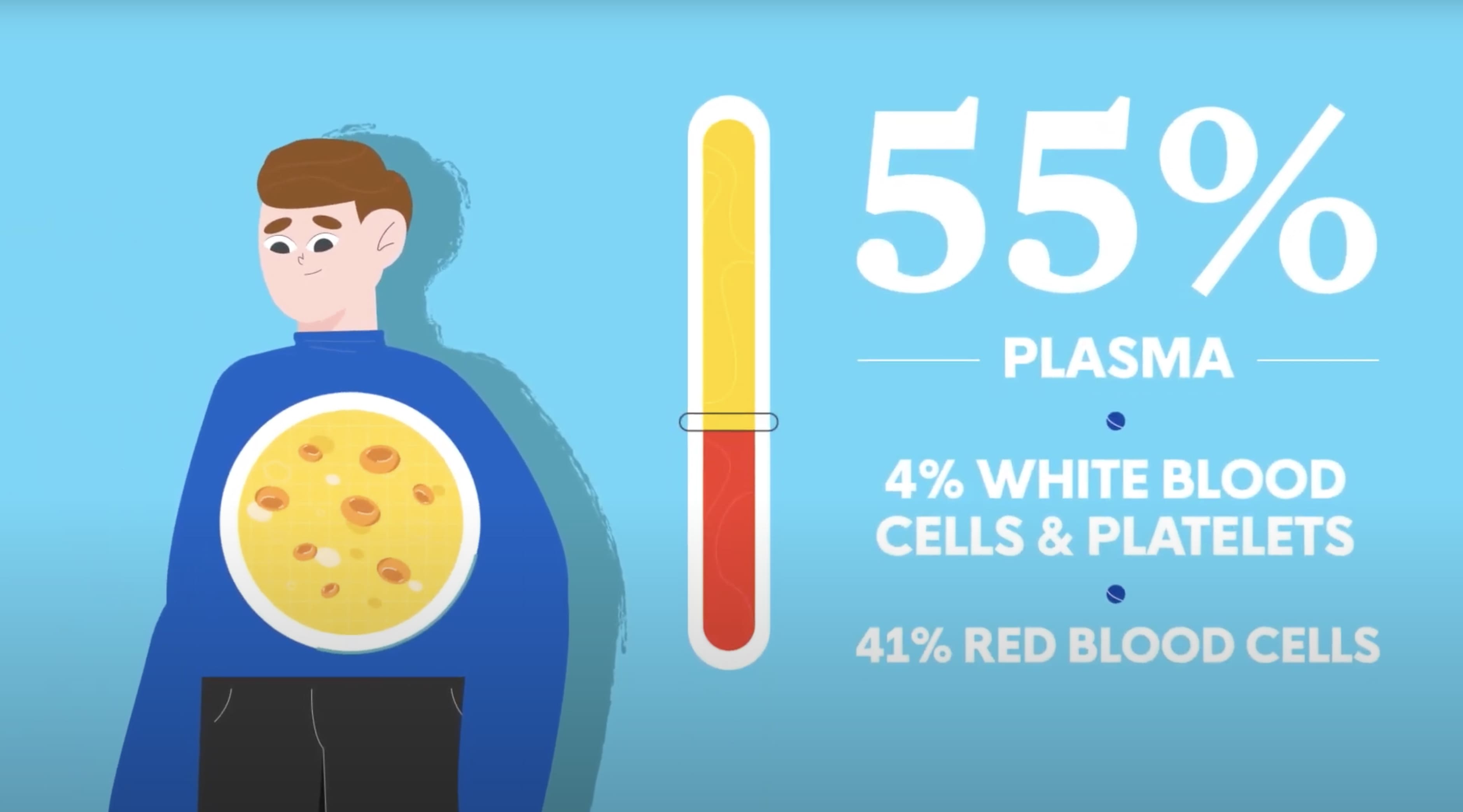
Differentiating Plasma from Blood
U.S. Roundtable on Immunoglobulin Use
EU BTC revision – The socioeconomic benefits of plasma-derived medicines
Why is Donating Plasma Important?
Strengthening the EU Blood Directive for patients who need plasma-derived medicines
Meet Amber, a Teacher and Dedicated Plasma Donor
Europe needs to collect more plasma – Improving patient access to plasma-derived medicines
EU BTC revision – Facts about compensating plasma donors
Highlights from the April 2021 Online Roundtable hosted by MEP Sirpa Pietikäinen (EPP, Finland)
April 2021 Online Roundtable hosted by MEP Sirpa Pietikäinen (EPP, Finland)
“Introduction to the Global Journey of Plasma" webinar
Highlights from the EP roundtable event “Ensuring Appropriate Patient Access to PDMPs”
Presentation of the 2020 Joachim Hilfenhaus Award
Presentation of the 2020 Robert W. Reilly Award
Keynote Address – Stella Kyriakides, European Commissioner for Health and Food Safety
Keynote Address – Admiral Brett Giroir, MD, U.S. HHS Assistant Secretary for Health
Patient Perspectives on COVID-19 and Access to Care
Plasma: An Overview
Experts on Coronavirus: The Virus, The Disease, Prevention and Treatment
Combating COVID-19 Industry Spotlight: Kedrion
Welcome to the Global Plasma Summit
Plasma 101
Combating COVID-19 Industry Spotlight: Grifols
Combating COVID-19 Industry Spotlight: CoVIg-19 Plasma Alliance
Combating COVID-19 Industry Spotlight: Emergent
Spotlight on Plasma Part One: Together We Are Stronger
Ensuring Appropriate Patient Access to Plasma-Derived Medicinal Products
PPTA webinar on unique patient access challenges to plasma-derived medicinal products in COVID times link
Thank you, plasma donors!
From PPTA: "Thank you, plasma donors!"
Prof. Lieven Annemans Speaks about the Value of Plasma-Derived Therapies
The Power of Plasma Donation - Hungarian
The Power of Plasma Donation - Spanish
The Power of Plasma Donation - Czech
The Power of Plasma Donation - German
The Power of Plasma Donation Animation - English
PPTA Welcomes European Parliament SANT Committee’s Approach to CMA Scope
.png)
PPTA Welcomes European Parliament SANT Committee’s Approach to CMA Scope
PPTA Welcomes SFEE’s Position on Plasma-Derived Medicines for Greece
.png)
PPTA Welcomes SFEE’s Position on Plasma-Derived Medicines for Greece
Plasma Powers Possibility: International Plasma Awareness Week Returns October 6-10
.png)
Plasma Powers Possibility: International Plasma Awareness Week Returns October 6-10
PPTA Welcomes Recognition of Pharmaceutical Supply Chain Diversity in EP Rapporteur’s Draft Report on the Critical Medicines Act
PPTA Welcomes Recognition of Pharmaceutical Supply Chain Diversity in EP Rapporteur’s Draft Report on the Critical Medicines Act
Plasma Protein Forum: Navigating the Winds of Change
Plasma Protein Forum: Navigating the Winds of Change
PPTA Responds to Recent Approval of Recombinant Albumin in China

PPTA Responds to Recent Approval of Recombinant Albumin in China
EPA: Czech Registry is Positive Step in Ensuring Donor Safety

EPA: Czech Registry is Positive Step in Ensuring Donor Safety
Marilena Vrana Selected to Lead PPTA’s European Efforts

Marilena Vrana Selected to Lead PPTA’s European Efforts
PPTA Statement on the 22nd Edition of the EDQM Blood Guide

PPTA Statement on the 22nd Edition of the EDQM Blood Guide
Professor Isabelle Meyts Honored with the 2025 Joachim Hilfenhaus Award

Professor Isabelle Meyts Honored with the 2025 Joachim Hilfenhaus Award
IPPC 2025 Set to Honor Two Decades of Progress Within a Critical Global Plasma Supply Chain

IPPC 2025 Set to Honor Two Decades of Progress Within a Critical Global Plasma Supply Chain
National and EU Stockpiling Requirements Do Not Ensure Equitable Access to Plasma-Derived Medicinal Products

National and EU Stockpiling Requirements Do Not Ensure Equitable Access to Plasma-Derived Medicinal Products
Plasma Protein Forum Prepares to “Taking Steps Toward a Better Tomorrow”

Plasma Protein Forum Prepares to “Taking Steps Toward a Better Tomorrow”
Sharing the Lifesaving Impact of Plasma Donation During International Plasma Awareness Week 2024

Sharing the Lifesaving Impact of Plasma Donation During International Plasma Awareness Week 2024
Monkeypox Virus and Plasma-derived Medicinal Products

Monkeypox Virus and Plasma-derived Medicinal Products
PPTA Welcomes EMA Regulatory Update on vCJD

PPTA Welcomes EMA Regulatory Update on vCJD
PPTA Welcomes Sharon Pearce as New Vice President, Lead of Government Affairs

PPTA Welcomes Sharon Pearce as New Vice President, Lead of Government Affairs
PPTA Regards SoHO Adoption a Step Forward to Strengthen Donor Protection and Plasma Supply in the EU

PPTA Regards SoHO Adoption a Step Forward to Strengthen Donor Protection and Plasma Supply in the EU
Dr. Charlotte Cunningham-Rundles Wins 2024 Joachim Hilfenhaus Award for Immunology Excellence

Dr. Charlotte Cunningham-Rundles Wins 2024 Joachim Hilfenhaus Award for Immunology Excellence
PPTA Responds to SoHO Regulation Compromise

PPTA Responds to SoHO Regulation Compromise
PPTA’s New Staff Members Aim to Further Plasma Sector Innovation and Advocacy

PPTA’s New Staff Members Aim to Further Plasma Sector Innovation and Advocacy
Summary of the PPTA/FDA Liaison Meeting (November 8, 2023)

Summary of the PPTA/FDA Liaison Meeting (November 8, 2023)
PPTA Statement on Issuance of the EU Critical Medicines List

PPTA Statement on Issuance of the EU Critical Medicines List
PPTA Statement and Reflection on the Quebec Biovigilance Committee’s 8th Biovigilance Forum

PPTA Statement and Reflection on the Quebec Biovigilance Committee’s 8th Biovigilance Forum
PPTA Statement on the Council’s negotiating mandate on the SoHO Regulation

PPTA Statement on the Council’s negotiating mandate on the SoHO Regulation
European Parliament Misses Opportunity to Increase Plasma Collection in Europe to Make Essential Medicines

European Parliament Misses Opportunity to Increase Plasma Collection in Europe to Make Essential Medicines
Study Confirms that Frequency of Source Plasma Donation as Regulated by U.S. FDA Does Not Impair Donor Health and Well-Being

Study Confirms that Frequency of Source Plasma Donation as Regulated by U.S. FDA Does Not Impair Donor Health and Well-Being

PPTA Press Release on the EU Parliament's ENVI Committee Vote on the SoHO Regulation

PPTA Press Release on the EU Parliament's ENVI Committee Vote on the SoHO Regulation
PPTA Statement on the Revision of the General EU Pharmaceutical Legislation

PPTA Statement on the Revision of the General EU Pharmaceutical Legislation

Announcing PPTA’s New Global Chair

Announcing PPTA’s New Global Chair
Monkeypox Virus and Plasma Protein Therapies

Monkeypox Virus and Plasma Protein Therapies
PPTA Executive Summary of FDA’s U.S. Immunoglobulin Utilization Study Report

PPTA Executive Summary of FDA’s U.S. Immunoglobulin Utilization Study Report
PPTA Statement on Review of Donor Blood Pressure Requirements

PPTA Statement on Review of Donor Blood Pressure Requirements
PPTA Statement on Review of Donor Weight Loss Monitoring

PPTA Statement on Review of Donor Weight Loss Monitoring
Statement on Update on Safety of Albumin

Statement on Update on Safety of Albumin
PPTA Statement on Safety of Plasma Protein Therapies and the H5N1 Viruses

PPTA Statement on Safety of Plasma Protein Therapies and the H5N1 Viruses
Statement on the Safety of Plasma Protein Therapies and Swine Influenza Virus

Statement on the Safety of Plasma Protein Therapies and Swine Influenza Virus
Human Parvovirus PARV4 and plasma protein therapies

Human Parvovirus PARV4 and plasma protein therapies
Ebola Virus and Plasma Protein Therapies

Ebola Virus and Plasma Protein Therapies
PPTA Statement on MSM Donor Policy

PPTA Statement on MSM Donor Policy
Zika Virus and Plasma Protein Therapies

Zika Virus and Plasma Protein Therapies
Plasma protein product safety and Creutzfeldt-Jakob disease (CJD)
.webp)
Plasma protein product safety and Creutzfeldt-Jakob disease (CJD)
PPTA Statement on “How Blood-Plasma Companies Target the Poorest Americans”

PPTA Statement on “How Blood-Plasma Companies Target the Poorest Americans”
New Coronavirus (SARS-CoV-2) and the Safety Margins of Plasma Protein Therapies
%20and%20the%20Safety%20Margins%20of%20Plasma%20Protein%20Therapies.webp)
New Coronavirus (SARS-CoV-2) and the Safety Margins of Plasma Protein Therapies
A Note to U.S. Plasma Donors on the Spread of Coronavirus

A Note to U.S. Plasma Donors on the Spread of Coronavirus
PPTA Responds to The Guardian Article

PPTA Responds to The Guardian Article
PPTA Statement on FDA Guidance Documents

PPTA Statement on FDA Guidance Documents
PPTA Repeats Appeals for Plasma Donations

PPTA Repeats Appeals for Plasma Donations
New York and California recognize the importance of plasma donation

New York and California recognize the importance of plasma donation
Plasma Donors Should be Celebrated, not Denigrated

Plasma Donors Should be Celebrated, not Denigrated
PPTA Statement on the Urgent Need for Plasma Donation

PPTA Statement on the Urgent Need for Plasma Donation
PPTA Statement on Alberta’s Passing of the Voluntary Blood Donation Repeal Act

PPTA Statement on Alberta’s Passing of the Voluntary Blood Donation Repeal Act
Yes, you can donate source plasma after getting the COVID vaccine

Yes, you can donate source plasma after getting the COVID vaccine
PPTA Statement on Immunoglobulin Use to Meet Clinical Need

PPTA Statement on Immunoglobulin Use to Meet Clinical Need
Human Plasma Donations Remain Important During COVID-19 Pandemic

Human Plasma Donations Remain Important During COVID-19 Pandemic
New position statement by CHMP regarding donor deferral criteria for sexual behaviour helps assessment of PMFs

New position statement by CHMP regarding donor deferral criteria for sexual behaviour helps assessment of PMFs
Study Shows Immunoglobulin Therapy is “Critical and Cost-Effective” in Treating COVID

Study Shows Immunoglobulin Therapy is “Critical and Cost-Effective” in Treating COVID
PPTA Responds to ProPublica Article

PPTA Responds to ProPublica Article
Plasma donations remain disappointingly low through ongoing pandemic, risking patients’ lives

Plasma donations remain disappointingly low through ongoing pandemic, risking patients’ lives
PPTA Disagrees with Ruling from Romanian Competition Council

PPTA Disagrees with Ruling from Romanian Competition Council
PPTA Leadership Update – Amy Efantis

PPTA Leadership Update – Amy Efantis
PPTA Statement on the Ukraine Crisis

PPTA Statement on the Ukraine Crisis
PPTA Statement on Report of ENVI Rapporteur for the SoHO Regulation

PPTA Statement on Report of ENVI Rapporteur for the SoHO Regulation
PPTA Statement on the draft EU Substances of Human Origin Regulation

PPTA Statement on the draft EU Substances of Human Origin Regulation
PPTA Recognizes First Plasma Collection Centers Certified in Hungary

PPTA Recognizes First Plasma Collection Centers Certified in Hungary
PPTA Recognizes World Hemophilia Day 2014

PPTA Recognizes World Hemophilia Day 2014
International Plasma Awareness Week to Celebrate Donors and Spotlight Rare Diseases

International Plasma Awareness Week to Celebrate Donors and Spotlight Rare Diseases
Ensuring Access to Medicines for Patients

Ensuring Access to Medicines for Patients
PPTA Recognizes 8th Annual International Rare Disease Day

PPTA Recognizes 8th Annual International Rare Disease Day
PPTA Recognizes World Hemophilia Day–April 17, 2015

PPTA Recognizes World Hemophilia Day–April 17, 2015
Alabama Lowers Minimum Donation Age to 18

Alabama Lowers Minimum Donation Age to 18
PPTA Recognizes Essential Contributions of Plasma Donors

PPTA Recognizes Essential Contributions of Plasma Donors
PPTA Names Amy Efantis President & CEO

PPTA Names Amy Efantis President & CEO
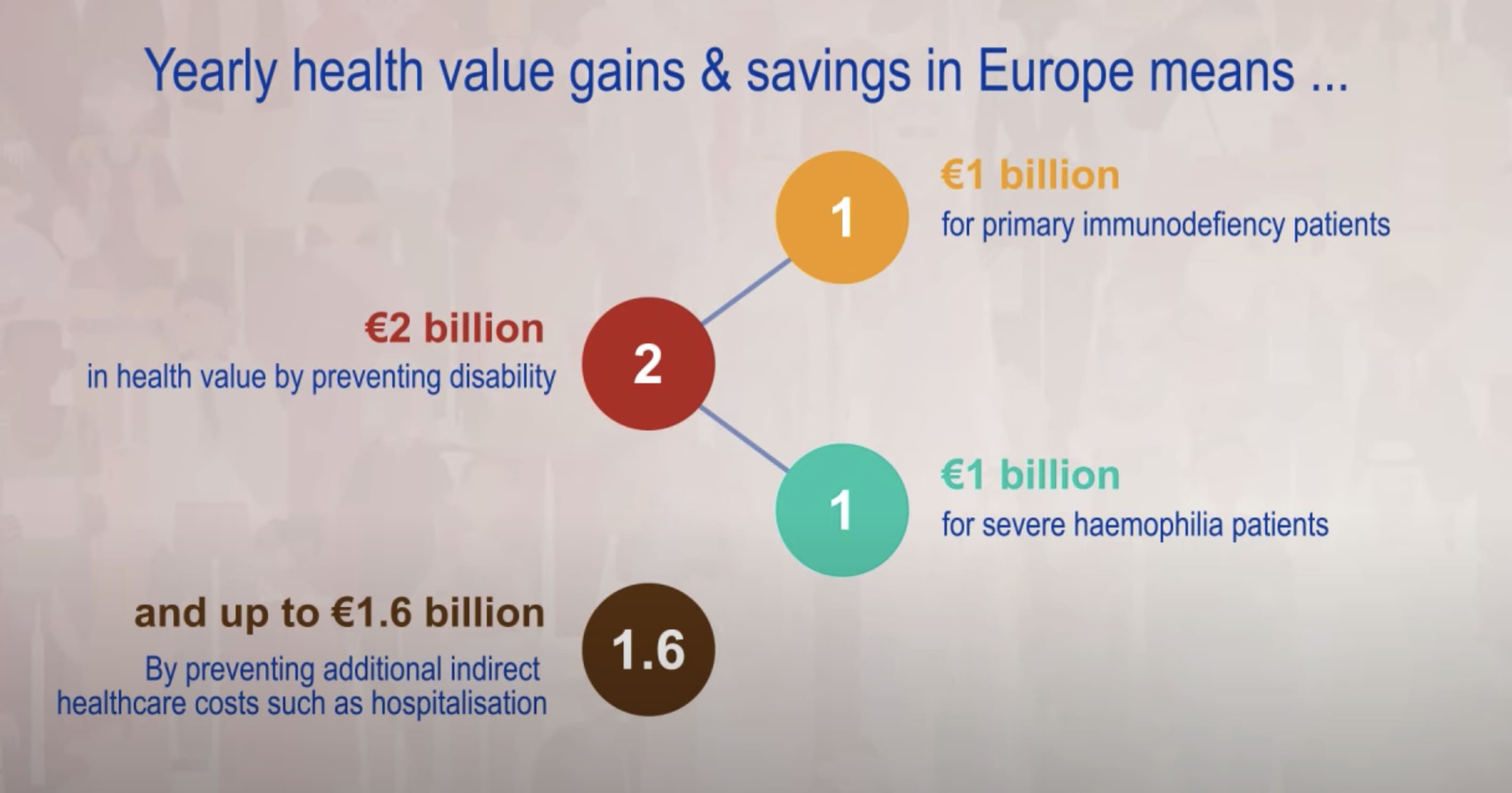
Plasma-Derived Therapies Provide Significant Health Value Gain

Plasma-Derived Therapies Provide Significant Health Value Gain
EU Health Commissioner Says Plasma Donation is “More Important Than Ever”
EU Health Commissioner Says Plasma Donation is “More Important Than Ever”
International Plasma Awareness Week 2020: Be a Hero, Donate Plasma Today

International Plasma Awareness Week 2020: Be a Hero, Donate Plasma Today
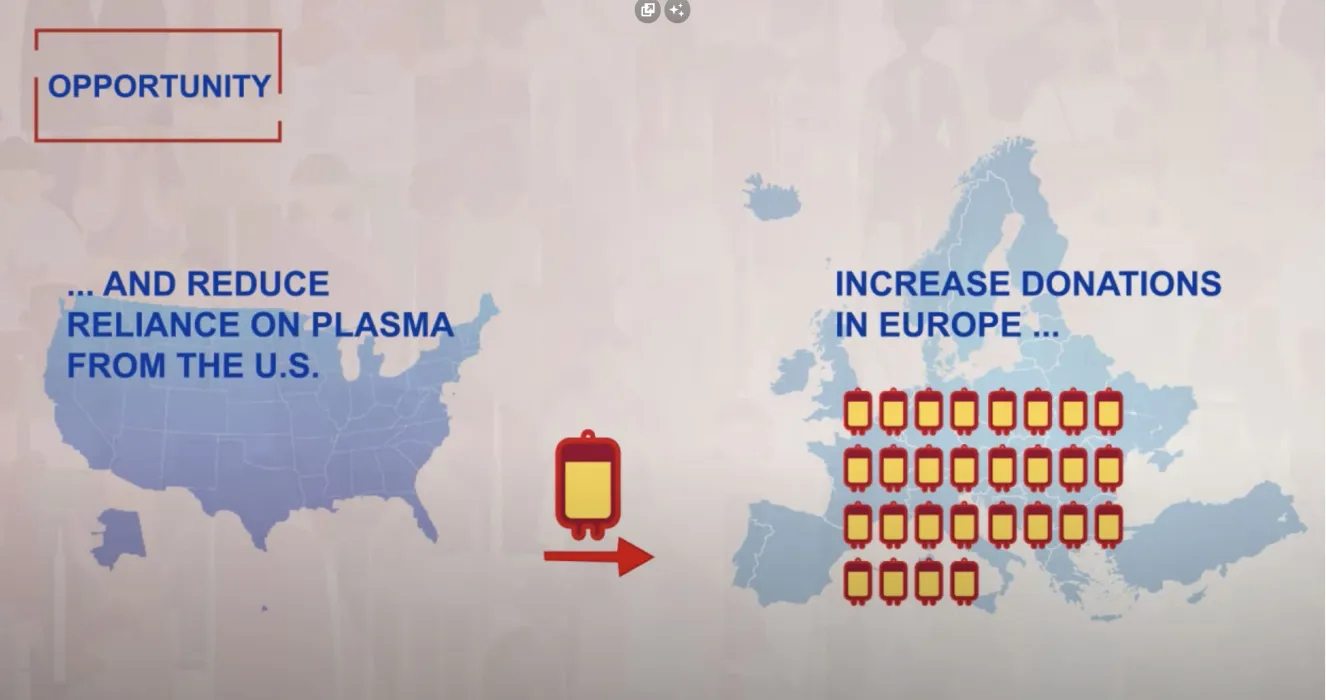
Inception Impact Assessment Underlines the Need for Timely Action to Decrease the Reliance on Third Countries for Plasma

Inception Impact Assessment Underlines the Need for Timely Action to Decrease the Reliance on Third Countries for Plasma
Lifting of long-time ban on use of UK plasma for manufacturing of immunoglobulins will help patients

Lifting of long-time ban on use of UK plasma for manufacturing of immunoglobulins will help patients
EU Health Commissioner says plasma donation plays a vital role

EU Health Commissioner says plasma donation plays a vital role
International Plasma Awareness Week 2021: Donate Plasma. Save Lives.

International Plasma Awareness Week 2021: Donate Plasma. Save Lives.
Calling on EU Policymakers to Increase Plasma Donations

Calling on EU Policymakers to Increase Plasma Donations
Adoption of the EP report “EU Pharmaceutical Strategy”

Adoption of the EP report “EU Pharmaceutical Strategy”
Plasma is Life podcast series episode 6: Peter Jaworski, Georgetown University
Plasma is Life podcast series episode 5: Nancy Di Salvo, GBS|CIDP Foundation
Plasma is Life podcast series episode 4: Vicki & Fred Modell, Co-Founders, Jeffrey Modell Foundation
Plasma is Life podcast series episode 2: U.S. Election 2020 Wrap-Up
Research Poster Highlights European Reimbursement and Access Policies in Glasgow
.png)
Research Poster Highlights European Reimbursement and Access Policies in Glasgow
Plasma Powers Possibility: How the Theme of IPAW Continues Year-Round as Donors Turn Science into Hope
.png)
Plasma Powers Possibility: How the Theme of IPAW Continues Year-Round as Donors Turn Science into Hope
Voices of Hope: Patients Share the Power of Plasma Donations
.png)
Voices of Hope: Patients Share the Power of Plasma Donations
Plasma Powers Possibility: IPAW 2025 Brings Global Attention to Plasma Donation and Patient Access
.png)
Plasma Powers Possibility: IPAW 2025 Brings Global Attention to Plasma Donation and Patient Access
International Plasma Awareness Week: Plasma is a Critical Lifeline for Patients
.png)
International Plasma Awareness Week: Plasma is a Critical Lifeline for Patients
International Plasma Awareness Week is Almost Here!
International Plasma Awareness Week is Almost Here!
Read PPTA’s 2025 Mid-Year Impact Report

Read PPTA’s 2025 Mid-Year Impact Report
What Does It Mean To Be Both a Caregiver and An Advocate?

What Does It Mean To Be Both a Caregiver and An Advocate?
Experience IPPC’s 20th Anniversary as if you were seated in the front row!

Experience IPPC’s 20th Anniversary as if you were seated in the front row!
Step Inside the 20th International Plasma Protein Congress: Central Theme, Speakers, and What’s New

Step Inside the 20th International Plasma Protein Congress: Central Theme, Speakers, and What’s New
"It's like a family reunion" - IPPC Attendees Share What Makes the Congress Special

"It's like a family reunion" - IPPC Attendees Share What Makes the Congress Special
Maximize Your Experience at the 20th International Plasma Protein Congress in Warsaw

Maximize Your Experience at the 20th International Plasma Protein Congress in Warsaw
Changing Minds, Changing Lives: The Importance of Raising Awareness for Plasma Donation

Changing Minds, Changing Lives: The Importance of Raising Awareness for Plasma Donation
The Value of PPTA Membership In An Evolving Industry: A Conversation with David Bell & Anita Brikman

The Value of PPTA Membership In An Evolving Industry: A Conversation with David Bell & Anita Brikman
The Problem with Mandatory Stockpiling of Plasma-Derived Medicines in the European Union

The Problem with Mandatory Stockpiling of Plasma-Derived Medicines in the European Union
Share Your #IPAW2024 Feedback

Share Your #IPAW2024 Feedback
IPAW Reflections: Words of Appreciation for the Lifesaving Impact of Plasma Donors

IPAW Reflections: Words of Appreciation for the Lifesaving Impact of Plasma Donors
Voices of Health Professionals in Treating Patients with Plasma-Derived Medicines

Voices of Health Professionals in Treating Patients with Plasma-Derived Medicines
IPAW In Focus: Prioritizing Donor Safety

IPAW In Focus: Prioritizing Donor Safety
Putting Advocacy In Action During IPAW and Beyond

Putting Advocacy In Action During IPAW and Beyond
Access to Plasma-Derived Medicines: A Lifesaving Resource for Patients

Access to Plasma-Derived Medicines: A Lifesaving Resource for Patients

Share Powerful Stories During #IPAW2024

Share Powerful Stories During #IPAW2024

Decorate Your Space for #IPAW2024!

Decorate Your Space for #IPAW2024!
Stay Connected on Social Media During #IPAW2024

Stay Connected on Social Media During #IPAW2024
Visit PlasmaWeek.org for Free IPAW Resources and More!

Visit PlasmaWeek.org for Free IPAW Resources and More!
Navigating Challenges and Opportunities in the Plasma Protein Therapeutics Industry: A Discussion with Anita Brikman and Giles Platford

Navigating Challenges and Opportunities in the Plasma Protein Therapeutics Industry: A Discussion with Anita Brikman and Giles Platford

A Mid-Year Message from PPTA President and CEO Anita Brikman

A Mid-Year Message from PPTA President and CEO Anita Brikman
Sharing the Power of Plasma on Capitol Hill
Sharing the Power of Plasma on Capitol Hill
From Patient to Provider: Laura’s Story

From Patient to Provider: Laura’s Story

2023 Annual Report

2023 Annual Report
PPTA Unveils Program for IPPC 2024

PPTA Unveils Program for IPPC 2024

A Story of Alpha-1 Antitrypsin Deficiency: Dan Coffin's Journey of Finding Hope, One Plasma Donation at a Time

A Story of Alpha-1 Antitrypsin Deficiency: Dan Coffin's Journey of Finding Hope, One Plasma Donation at a Time
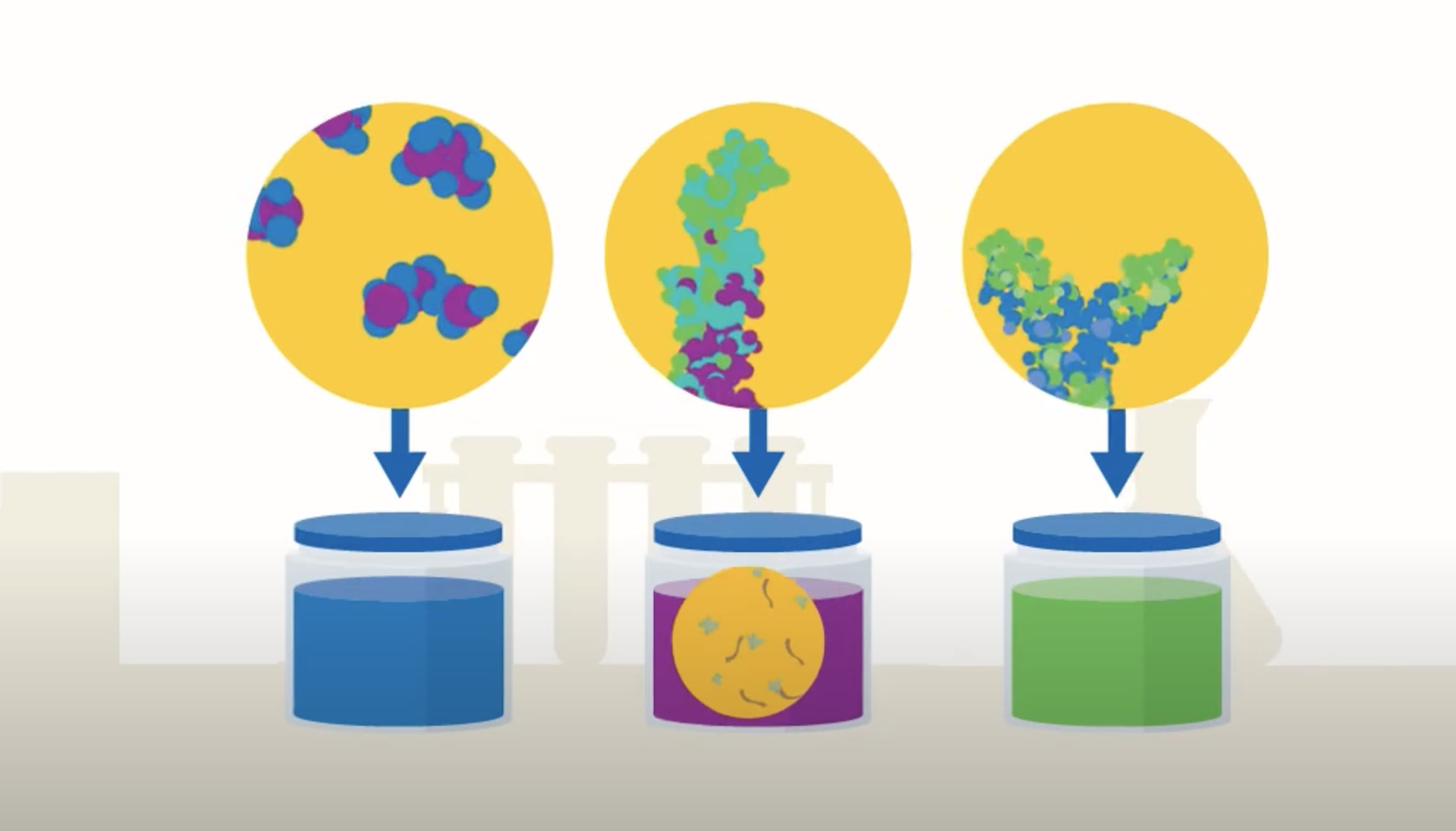
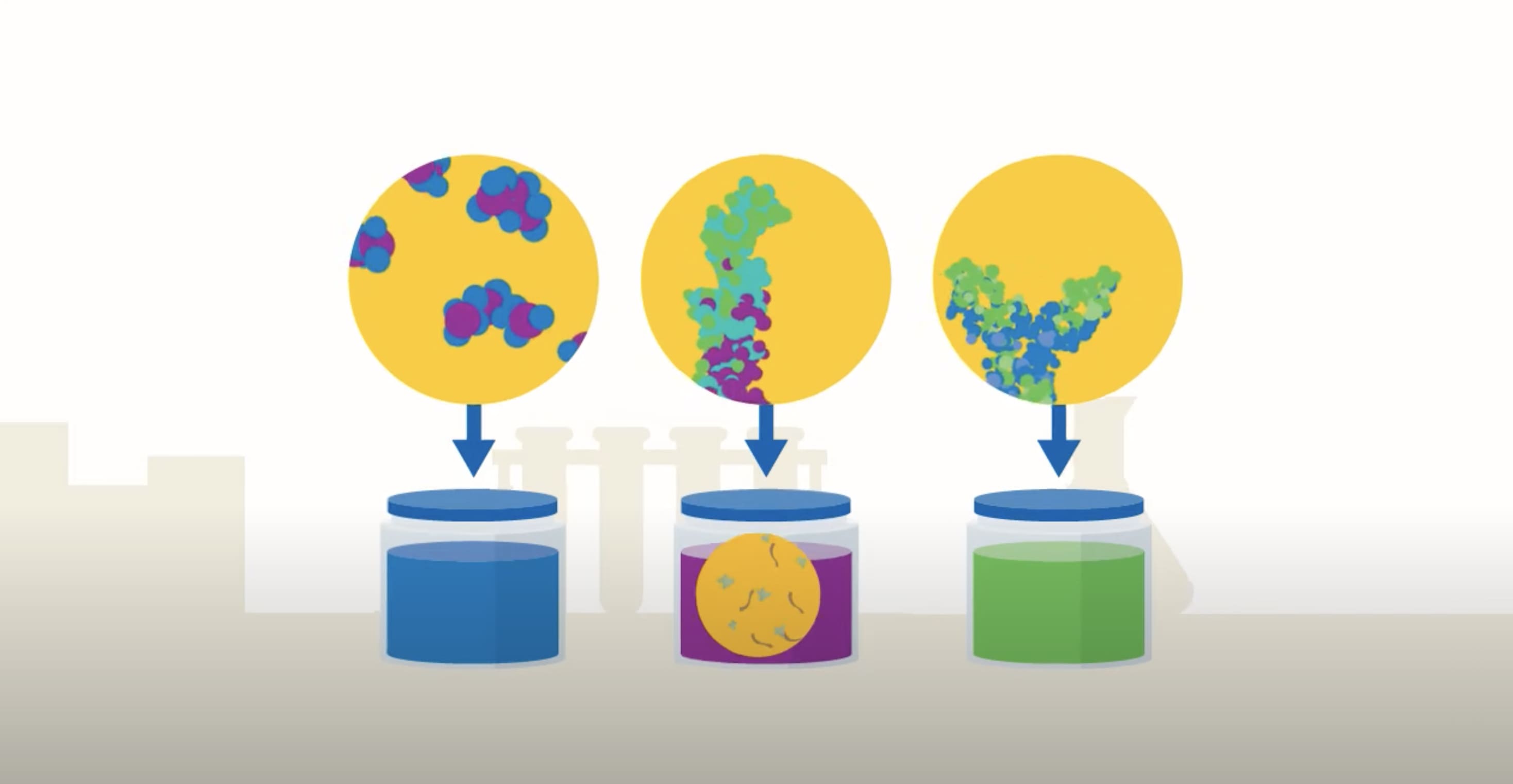
Stepping Inside a Plasma Fractionation Plant: How Human Plasma Becomes Life-Saving Medicine

Stepping Inside a Plasma Fractionation Plant: How Human Plasma Becomes Life-Saving Medicine
Events
National Kawasaki Disease Awareness Day
National Kawasaki Disease Awareness Day
Join PPTA
Become a member of PPTA! PPTA engages with stakeholders to establish and improve regulations and policies that affect the sector.
By being a member of our industry-leading association, you can work closely with influential partners, enjoy numerous benefits and access useful resources.


















Contact us
If you have any inquiries, get in touch with us by filling out our contact form linked below.
Members of the media are encouraged to send press inquiries to media@pptaglobal.org



















%201.jpg)











-%20Day%20One.webp)
-%20Day%20Two.jpg)

























.jpg)